Spermidine Promotes Human Hair Growth and Is a Novel Modulator of Human Epithelial Stem Cell Functions
Introduction
Polyamines (spermidine, putrescine and spermine) are multifunctional polycationic aliphatic amines, which serve crucial roles in cell survival. Besides serving as nutrients and metabolic regulators [1,2], polyamines have been implicated as mediators of key cell functions, such as proliferation, migration and differentiation [3–7]. Polyamines also stabilize DNA/RNA and modulate DNA replication/transcription [1,2,8–10], and stabilize membrane and cytoskeletal proteins [11,12]. Recently a key polyamine, spermidine, has even been hailed as a new longevity agent due to its impact on chromatin-mediated regulation of gene expression [13]. While polyamines are indispensable for cell proliferation, and are needed for the growth of rapidly regenerating tissues and tumors [14,15], the full spectrum of functions of spermidine in normal human tissue physiology remains poorly understood [16]. The hair follicle (HF) is one of the most highly proliferative organs in mammalian biology [17,18]. Therefore, polyamines have long been suspected to be important for hair growth [19]. For example, inhibiting polyamine synthesis significantly modulates murine hair growth [20], and polyamines have an essential role in determining sheep HF growth and diameter [21]. In addition, a recent study has shown that a topical administration of amethylspermidine, a stable analogue of spermidine, induced hair growth in telogen phase mice [22]. Surprisingly, however, studies utilizing several transgenic mice lines with altered polyamine metabolism [6,19,20,23–28] showed that the most prominent phenotype of these mice was hair loss due to disturbed proliferation of follicular keratinocytes.
Introduction
Polyamines (spermidine, putrescine and spermine) are multifunctional polycationic aliphatic amines, which serve crucial roles in cell survival. Besides serving as nutrients and metabolic regulators [1,2], polyamines have been implicated as mediators of key cell functions, such as proliferation, migration and differentiation [3–7]. Polyamines also stabilize DNA/RNA and modulate DNA replication/transcription [1,2,8–10], and stabilize membrane and cytoskeletal proteins [11,12]. Recently a key polyamine, spermidine, has even been hailed as a new longevity agent due to its impact on chromatin-mediated regulation of gene expression [13]. While polyamines are indispensable for cell proliferation, and are needed for the growth of rapidly regenerating tissues and tumors [14,15], the full spectrum of functions of spermidine in normal human tissue physiology remains poorly understood [16]. The hair follicle (HF) is one of the most highly proliferative organs in mammalian biology [17,18]. Therefore, polyamines have long been suspected to be important for hair growth [19]. For example, inhibiting polyamine synthesis significantly modulates murine hair growth [20], and polyamines have an essential role in determining sheep HF growth and diameter [21]. In addition, a recent study has shown that a topical administration of amethylspermidine, a stable analogue of spermidine, induced hair growth in telogen phase mice [22]. Surprisingly, however, studies utilizing several transgenic mice lines with altered polyamine metabolism [6,19,20,23–28] showed that the most prominent phenotype of these mice was hair loss due to disturbed proliferation of follicular keratinocytes.
In human skin, topical application of eflornithine (difluoromethylornithine, DFMO), an inhibitor of ornithine decarboxylase (ODC), the rate limiting enzyme in the polyamine biosynthesis pathway [29], can reduce undesired, excessive hair growth [30,31]. We have previously shown that addition of DFMO to organ-cultured human scalp HFs shortens the growth phase of the hair cycle (anagen) and inhibits hair shaft production, accompanied by a decrease in matrix keratinocyte proliferation [32]. However, whether and how spermidine itself affects human hair growth directly is unknown. Therefore, we have exploited the HF as an excellent model system for exploring physiological polyamine functions in human skin [29]. Specifically, we have studied in normal, microdissected human scalp HFs under serum-free organ culture conditions [33– 35] whether spermidine impacts on basic hair biology parameters. We opted for testing the spermidine doses that have been previously shown to modulate wool follicles growth in organ culture [21], and that correspond to the physiological spermidine levels in human plasma [36]. Using keratin 15 (K15) expression and K15 promoter-driven green fluorescent protein (GFP) expression as a system for assessing human epithelial HF stem cell functions in situ and in vitro [35,37,38], we also explored whether spermidine alters human HF epithelial stem cell clonogenicity, whose modulation by polyamines is as yet unknown [17,18,38,39].
Results
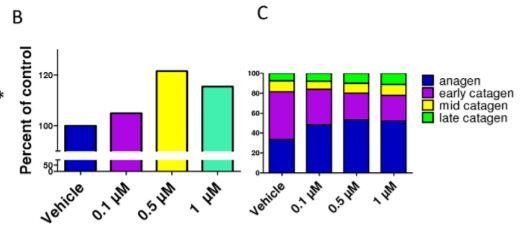
Spermidine stimulates hair-shaft elongation and prolongs anagen
rolongs anagen Administration of spermidine for 6 days slightly, but significantly, increased hair shaft growth of microdissected, organcultured normal human scalp HFs (Figure 1A). This effect was maximal and significant at 0.5 mM, and led to more than a 20% increase in hair shaft production after 6 days in culture (Figure 1B). However, whether spermidine can also prolong the duration of anagen is clinically much more important than the effect on hair elongation since this directly impacts on the amount of hair that is shed (e.g., a reduced percentage of HFs in anagen, clinically, will inevitably result in a higher percentage of catagen and subsequently telogen HFs, thus leading to telogen effluvium – and vice versa) [17,18,40]. To assess this, quantitative hair cycle histomorphometry was performed [41,42]. Indeed, after 6 days of organ culture, all doses of spermidine investigated led to an increase in the percentage of HFs in anagen, and decreased that of catagen HFs (Figure 1C). In the presence of spermidine, only 47– 52% of the HFs spontaneously entered catagen, while 67% of the control HFs had already done so.
Spermidine downregulates ODC expression in the HF
permidine downregulates ODC expression in the HF ODC, the rate-limiting enzyme of polyamine synthesis, reportedly is expressed in highly proliferative hair matrix keratinocytes [29,43]. We have also demonstrated that ODC is present in the matrix keratinocytes of human anagen VI HFs (Fig. 2A). Since polyamines negatively regulate ODC activity, both on transcriptional and post-transcriptional level [29], we checked whether any such effect is also apparent in spermidine-treated HFs.
We found that spermidine treatment for 6 days downregulated ODC protein expression in situ (Figure 2B), and showed a tendency towards decreased ODC mRNA transcription after 24 h treatment (Figure 2C), though the latter was not significant. However, in isolated, cultured human HF-derived outer root sheath (ORS) keratinocytes, 0.5 mM spermidine treatment for 48 h significantly downregulated ODC mRNA expression (Figure 2D). Together, these data suggest that excess spermidine induces intracellular counter-regulatory events in human HF epithelium in situ through which further intrafollicular polyamine synthesis is restricted by inhibiting ODC gene and protein expression.
Spermidine stimulates human hair matrix and epidermal keratinocyte proliferation
The fact that spermidine prolonged anagen suggested effects on highly proliferative hair matrix keratinocytes [17,18]. In situ, spermidine slightly, but not significantly stimulated hair matrix keratinocyte proliferation, as assessed by quantitative Ki67- immunohistomorphometry (Figure 3A), and did not significantly alter hair matrix keratinocyte apoptosis (Figure 3A). However, fluorimetric proliferation assays revealed that spermidine dosedependently stimulated the proliferation of cultured primary human epidermal keratinocytes (Figure 2B). FACS analysis revealed that spermidine promoted the accumulation of cells in the S/G2-M phases of the cell cycle (data not shown). Thus, the promotion of S/G2-M entry by human HF keratinocytes may be one potential mechanism by which spermidine may exert its anagen-prolonging effects.
Spermidine differentially modulates the gene expression profile of human HFs
In order to identify potential (direct or indirect) target genes of spermidine action in human HFs, a genome-wide microarray analysis was performed on two independent sets of organ-cultured human scalp HFs, derived from two healthy female volunteers (aged 47 and 67 yrs), which had been treated with 0.5 mM spermidine or vehicle for 24 h and 48 h, respectively. In order to check which genes are being constitutively activated following spermidine administration, we made a comparison between these two timepoints. Adopting rigid selection criteria, i.e. selecting only those genes whose intrafollicular transcription was modified .2-fold p , 0.05) into the same direction in both individuals, only five genes were identified as significantly and equidirectionally upregulated by the tested spermidine dose (Table 1). Of these spermidine-regulated genes, SYVN1 and NACA are known to be important for normal cell homeostasis and function, including endoplasmic reticulum-associated protein degradation [44] and folding and targeting of nascent proteins [45]. SLC25A3 encodes a mitochondrial phosphate carrier, essential for the aerobic synthesis of adenosine triphosphate [46], and POP3 is important for cell adherence and migration [47] (see Discussion for details).
Spermidine up-regulates expression of the epithelial stem cell-associated keratins K15 and K19, and modulates K15 promoter activity in situ
Spermidine up-regulates expression of the epithelial stem cell-associated keratins K15 and K19, and modulates K15 promoter activity in situ
Anagen maintenance requires constant production of HF keratinocytes from resident epithelial stem cells (eSCs) and their progeny, which are thought to migrate towards the hair matrix [18,48]. Since polyamines have been shown to be required for the migration [3,4] and differentiation of various progenitor cell populations [5–7,49] as well as for cell cycle progression [50,51], we therefore addressed whether spermidine also affects human HF eSCs. These cells are located primarily in the bulge and are characterized by expression of K15; however, K15+ progenitors are also found further down the ORS [17,18,38,39]. Even though our microarray analysis had not identified any HFassociated keratins as spermidine-regulated genes (possibly for methodological or sub-threshold reasons), quantitative immunohistomorphometry of K15 expression in situ clearly showed that spermidine significantly increased K15 immunoreactivity in the basal layer of the proximal ORS after 6 days (Figure 4A). To confirm this effect in isolated human HF keratinocytes, which are not influenced by the HF mesenchyme, HF melanocytes, and intrafollicular hematopoietic cells, we measured the steady-state levels of K15 mRNA in cultured human ORS keratinocytes that had been treated for 48 h with 0.5 mM spermidine. Indeed, K15 mRNA was upregulated after 48 h of treatment (Figure 4B). Conversely, ODC inhibition by DMFO significantly downregulated K15 expression after 6 days (Figure 4C). This suggests that K15 protein expression in situ is profoundly regulated by spermidine.
In the lower two doses tested, spermidine also significantly increased keratin 19 (K19) protein expression after 6 days, an independent human HF epithelial progenitor cell marker [35,52] (Figure 4D). Additionally, 0.5 mM spermidine administration led to increased K19 mRNA expression in organ-cultured HFs after 24 h (Figure 4E). Treatment of cultured ORS keratinocytes with 0.5 mM spermidine tended to upregulate K19 mRNA after 48 h, but this was not statistically significant (Figure 4F). To further explore the effect of spermidine on human HF eSCs in the bulge [39,48], we employed a novel human K15 promoterdriven GFP reporter assay, which demarcates K15+ human HF epithelial progenitor cells in situ [37,38]. This revealed that spermidine also significantly and dose-dependently modulated human K15 promoter activity in the bulge region in situ: As measured by the intensity of GFP fluorescence, at 0.5 mM spermidine up-regulated the transfected K15 promoter activity in situ, while it down-regulated it at 0.1 mM or 1 mM (Figure 5A).
Spermidine dose-dependently modulates colony forming efficiency and K15 expression of isolated K15-GFP+ cells in vitro
To further investigate how spermidine impacts on human HF epithelial progenitor cells in the absence of HF mesenchyme, HF melanocytes and intrafollicular hematopoietic cells, we isolated and cultured human HF K15-GFP+ progenitor cells [37,38]. These experiments showed that only the 0.5 mM spermidine dose significantly up-regulated the colony forming efficiency of K15- GFP+ progenitor cells (Figure 5B).
This spermidine dose also increased their K15 mRNA expression on days 3 and 6 (Figure 5C), and slightly enhanced their K15 protein expression on days 1 and 3 (Figure 5D). Furthermore, 0.5 mM spermidine also stimulated proliferation on days 3 and 6 of these isolated human HF progenitor cells in vitro (Figure 5D). In contrast, 1 mM spermidine significantly inhibited K15 mRNA expression and the proliferation activity of K15-GFP+ cells in vitro (Figures 5C, D). These in vitro results are comparable with the in situ observations, which also showed maximal spermidine growth-modulatory effects on the intact HF mini-organ at the same dose. Taken together, this suggests that the spermidine-induced growth-promotion of human HFs at 0.5 mM (Figures 1A, B) may be mediated, at least in part, by an effect on epithelial HF progenitor cells, which are also stimulated by the same dose.
Discussion
While it has long been postulated that polyamines may stimulate hair growth [22,29,53], the current study is the first to provide direct evidence that this is actually the case in human HFs. Specifically, we demonstrate that spermidine stimulates hair shaft elongation, accompanied by prolongation of anagen, and thus directly promotes human HF growth. This is in line with recent in vivo evidence that topically applied alpha-methylspermidine induces anagen in mouse telogen HFs [22].
The anagen prolongation/catagen inhibition by spermidine demonstrated here is clinically important: If our human HF organ culture data are transferrable to the in vivo situation, spermidine administration may help to counteract multiple forms of hair loss associated with excessive hair shedding. Namely, topical or nutraceutical spermidine application may reduce telogen effluvium in patients that suffer from hair loss due to premature anagen termination (e.g. by androgens, perifollicular inflammation, iron or estrogen deficiency, or effluvium-inducing drugs) [17,54]. ODC is expressed in the anagen hair matrix [29,55], and reducing intrafollicular spermidine synthesis by the inhibition of ODC activity reduces human hair shaft growth and shortens anagen duration [32]. Together with the anagen-prolonging and hair growth-promoting effects of spermidine demonstrated here, this suggests that intrafollicular synthesis of spermidine is important for keeping a HF in its growth stage. However, overexpression of ODC in mice results in the formation of dermal cysts, leading to the opposite effect ( = hair loss) [20,25]. This is postulated to result from the excessive accumulation of putrescine, which causes disturbed keratinocyte differentiation and increased proliferation. In fact, we show here that spermidine application downregulates intrafollicular ODC expression on the gene and protein levels (Fig. 2B-D), and may thus protect the human scalp HF against excessive synthesis of putrescine so as to avoid deleterious polyamine effects on human hair growth. To our surprise, although also evident in the proliferating keratinocytes of the hair bulb, the strongest expression of ODC was evident in the companion layer of the HF (Fig. 2A).
This expression pattern coincides with the intriguing pattern of expression of K6/K16, the keratins known to be expressed in highly proliferating cells [56]. Thus, our findings add to the mystery which still surrounds this layer in the HF, and which awaits further investigation. Despite the known general proliferation-stimulatory properties of polyamines [57,58], which were confirmed here in cultured human epidermal keratinocytes (Fig. 3B), and the demonstrated inhibitory effects of DFMO on human hair matrix keratinocytes [32], spermidine exerted only minor effects on hair matrix proliferation in situ. Therefore, much of the stimulatory effect of spermidine on hair shaft production appears to arise from a prolongation of anagen (Fig. 1C). This supports the concept that polyamines are potent regulators of HF cycling, not only in mice [19,22,26], but also in man. It also provides the first evidence that one defined polyamine, spermidine, alters HF cycling by direct effects on the HF which are independent of extrafollicular changes induced in the concentration of various polyamines and/or in the activity of their multiple different target genes. The recent provocative report that spermidine may promote longevity [13,59] may be relevant in the current context, if one considers that, with the notable exception of its pigmentary unit, the HF is one of the most strikingly aging-resistant organs of the human body [60]. Furthermore, the duration and prolongation of anagen is a very faithful indicator of HF vitality [32,61]. Therefore, one wonders whether spermidine impacts positively on a HF’s individual lifespan.
Since the latter vitally depends on its epithelial stem cell compartment [48,62], this raises the question whether the normal function of HF epithelial stem cells depends on proper intrafollicular spermidine synthesis and/or availability.Though our pilot study was not designed to provide definitive proof for the validity of this hypothesis, our current data suggest that human HF epithelial stem cells are profoundly modulated by spermidine: Spermidine not only upregulates K15 and K19 protein expression in situ (Fig. 4A,D), but intrafollicular spermidine synthesis is also needed for normal K15 expression (Figure 4C). Moreover, one tested spermidine dose (0.5 mM) stimulated K15 promoter activity and colony forming efficiency and long-term proliferation of isolated, primary human HF epithelial progenitors (Fig. 5A,B,D). Our observation that the highest tested dose of spermidine (1 mM) inhibited colony forming efficiency, K15 promoter activity, and proliferation at day 6, might well result from the metabolism of excess spermidine to toxic compounds that enhance oxidative damage, such as hydrogen peroxide [29]. Although strong upregulation of K15 was evident in all of our experiments at the 0.5 mM dose, K15 protein expression of K15- GFP+ cells in vitro was surprisingly downregulated on day 6 (Fig. 5D). This was in contrast to the upregulation of the corresponding mRNA expression that was observed at the same dose (Fig. 5C). It is possible that the rapid proliferation of the K15- GFP+ cells observed after 6 days of 0.5 mM spermidine administration (as assessed by Ki-67 immunoreactivity, Fig. 5D) did not allow enough time for the K15 protein to assemble in the cells, and to reach the level of detection.
It has also been shown before that polyamines have an effect on the post-translational regulation of proteins, affecting protein degradation by direct or indirect effect on proteases [63]. Therefore, changes in protein expression may not always correlate with the changes observed at the mRNA level. Our study presents the first evidence that spermidine is a novel determinant in human eSCs biology, most notably of K15 and K19 expression by primary human epithelial progenitor cells in situ and in vitro. These findings are in line with the prior demonstration that ODC is expressed in the bulge region of the HF [55,64], where it colocalizes with that of K15 and K19 expression [52]. While polyamines are known to affect the keratin composition of wool follicles [21], it was previously unknown that polyamines actually regulates the expression of human eSC-associated keratins. Moreover, we provide the first available evidence that inhibiting the key enzyme of polyamine synthesis (ODC) down-regulates K15 expression. Thus K15 expression in situ is profoundly regulated by spermidine, and both polyamines and ODC activity impact on the expression of this HF epithelial progenitor cell marker keratin. Our finding that ODC expression on the gene and protein level (and thus likely ODC activity) underlies a negative, dose-dependent feedback regulation by spermidine (Fig. 2B) underscores the apparent importance of keeping intrafollicular polyamine synthesis in check.That the highest dose of spermidine tested did not reduce ODC expression (Figure 1B) may suggest that adequate ODC activity remains needed as a part of the biological stress response to excessive spermidine levels.
We had hoped to obtain specific leads from our microarray analysis on how spermidine may exert its anagen-prolonging, stem cell-modulatory, and K15/K19-regulatory effects. While these results identified five novel intrafollicular candidate target genes for spermidine-mediated signaling (Table 1) that have not yet been investigated in the spermidine literature, these genes do not sufficiently explain the underlying mechanisms of action. However, the fact that the identified candidate genes are important for vital cell organelles and cell homeostasis fits well to the general concept that spermidine supports HF and eSC vitality. For example, synoviolin (coded by SYVN1) is a ubiquitin ligase, which plays an important role in endoplasmic reticulum-associated protein degradation [44], the NACA gene encodes the nascentpolypeptide-associated complex alpha polypeptide, a part of the protein translation chaperone complex [45], and SLC25A3 is a mitochondrial phosphate carrier, which is essential for the aerobic synthesis of adenosine triphosphate [65]. Particularly interesting potential spermidine target gene is POP3, which belongs to the highly conserved popeye domain-containing family, and has been implicated in cell adherence and migration [47]. Although these microarray results could not be further validated since all available HF samples and sections had been consumed for the analyses reported here, our preliminary data provide new leads to previously unsuspected (direct or indirect) spermidine target genes in human tissue physiology. An intriguing chance observation of our study was the finding that spermidine clearly up-regulated transcription of K77 in the HFs of two female patients (Table 1).
Since this keratin has previously been claimed to be exclusively expressed in eccrine glands, we are now following this lead up on the gene and protein level in order to obtain deeper insights into the unexpected and enigmatic functions that K77 may have in human HF biology, and why expression of this gene is so spermidine-sensitive. In summary, our study provides the first evidence that spermidine directly impacts on the growth, cycling, keratin expression and epithelial progenitor functions of human HFs. Due to its anagen-prolonging effects, spermidine deserves rigorous clinical testing as a candidate anti-hair loss agent. It could become an adjuvant therapy for hair loss disorders associated with premature catagen induction, leading to telogen effluvium, and/ or reduced hair shaft production. Moreover, we show that the complex regulatory role of polyamines in human epithelial biology in situ extends far beyond the mere stimulation of proliferation. Our study also documents that, to further dissect the full range of polyamine functions in normal human tissue physiology, human HF organ culture offers a highly instructive, clinically relevant research tool (34).
Materials and Methods
HF organ culture
Anagen VI HFs were microdissected from normal temporofrontal human scalp skin obtained after written informed patient consent from ten healthy adult females undergoing routine facelift surgery, adhering to the Helsinki guidelines and following approval by the Institutional Research Ethics Committee of the University of Lu¨beck.
In this clinically highly relevant in vitro-assay, amputated HFs in the growth phase of the hair cycle (anagen) continue for several days to produce a pigmented hair shaft at almost the normal speed of normal anagen HFs in vivo, and display cyclic growth activity in vitro by spontaneously entering into the regression phase of the hair cycle (catagen). For immunohistochemical studies, isolated HFs from four individuals were used for routine 6-day HF organ culture as previously described [17,18,32- 35]. Spermidine (0.1, 0.5 or 1 mM), DFMO (400 mg/l) or vehicle (distilled water) were administered once for each change of culture medium (every 48 h). After 6 days, all test and control HFs were embedded, snap-frozen, and processed for longitudinal cryosectioning. The frozen sections were stored at 280uC until used. HFs from one individual were used for a 24 h organ culture, and utilized for a genome-wide microarray essay and quantitative PCR (qPCR). HFs from four additional individuals were subjected to 48 h organ cultures. HFs from two individuals were used for K15 promoter-driven green fluorescent protein (GFP) expression construct transfection (see below). HFs from an additional one female patient were used for a genome-wide microarray essay. HFs from one individual were used for isolating and culturing of K15-GFP positive cells.