Spermidine-triggered autophagy ameliorates memory during aging
Polyamine levels decline with age not only in Drosophila but also in rodents and humans. Now, our observations suggest a causal connection between this decline and the onset of AMI: spermidine feeding restores polyamine levels in 30-d-old flies while at the same time it maintains short-term and intermediate-term memory performance comparable to that of young animals. Of note, spermidine treatment has been previously described to promote longevity and improve general health. However, its suppressing impact on AMI seemingly does not result from these rather generic effects but is apparently more specific. This specificity is reflected in the observations that spermidine feeding does not ameliorate young flies’ memory (but only “helps” old flies), it does not protect from age-increased locomotor impairment, nor does it influence another memory component, anesthesia-resistant memory, that is not subject to much of an age-induced decline. Spermidine seems to at least partly execute its protection from AMI via autophagy induction: its administration results in upregulated transcript levels of positive autophagy regulators in the brain, averts the age-related and detrimental decrease in autophagic clearance of ubiquitinated proteins in brain neuronal cells and blocks the age-associated decrease of Atg8a levels, with the latter representing a bona fide marker for autophagic activity.Importantly, in reverse, spermidine fails to suppress AMI in flies deficient for Atg7 or Atg8, both essential for autophagy in Drosophila, which is consistent with a causal connection between autophagy induction and AMI prevention. Of note, loss of only one Atg7 gene copy already occludes spermidine protection, indicating that effective autophagy induction is at the heart of the observed effects.
Polyamine levels decline with age not only in Drosophila but also in rodents and humans. Now, our observations suggest a causal connection between this decline and the onset of AMI: spermidine feeding restores polyamine levels in 30-d-old flies while at the same time it maintains short-term and intermediate-term memory performance comparable to that of young animals. Of note, spermidine treatment has been previously described to promote longevity and improve general health. However, its suppressing impact on AMI seemingly does not result from these rather generic effects but is apparently more specific. This specificity is reflected in the observations that spermidine feeding does not ameliorate young flies’ memory (but only “helps” old flies), it does not protect from age-increased locomotor impairment, nor does it influence another memory component, anesthesia-resistant memory, that is not subject to much of an age-induced decline. Spermidine seems to at least partly execute its protection from AMI via autophagy induction: its administration results in upregulated transcript levels of positive autophagy regulators in the brain, averts the age-related and detrimental decrease in autophagic clearance of ubiquitinated proteins in brain neuronal cells and blocks the age-associated decrease of Atg8a levels, with the latter representing a bona fide marker for autophagic activity.Importantly, in reverse, spermidine fails to suppress AMI in flies deficient for Atg7 or Atg8, both essential for autophagy in Drosophila, which is consistent with a causal connection between autophagy induction and AMI prevention. Of note, loss of only one Atg7 gene copy already occludes spermidine protection, indicating that effective autophagy induction is at the heart of the observed effects.
Consistently, these Atg7 heterozygotes start with normal memory scores, but show faster AMI. These effects in the fly brain are concordant with many reports showing that, on the one hand, spermidine treatment triggers protective autophagy in different model organisms and, on the other hand, autophagy is a crucial regulator of age-associated pathologies, including neurodegenerative disorders. In this respect, it remains interesting to explore which of the existing autophagy types might contribute to the protection from AMI and to which extent. While micro- and/or macroautophagic processes aimed at disposing of damaging polyubiquitinated proteins and thus at hindering their accumulation during aging seem to be at the core of protection, it is conceivable that other intracellular proteins and pathways acutely involved in age-dependent deterioration may be targeted. Mitochondria, for instance, can be specifically directed for autophagic disintegration by mitophagy and their dynamics, quality control and disposal are connected to diverse neurodegenerative diseases. In fact, neurons belong to the most energy-consuming cell types, suggesting that surveillance and clearance of damaged mitochondria may play a crucial role in neurodegenerative pathogenesis. However, it remains unexplored whether spermidine can induce mitophagy in general and upon AMI in particular, and further studies will have to clarify that. Interestingly, in mammals, there exists a crosstalk between macro- and chaperonemediated autophagy, a form of self-digestion that allows the lysosomal degradation of cytosolic proteins harboring a specific motif.
This rather selective disposal mechanism has been related to chronic neurodegenerative diseases, but its actual impact and possible targeting in the frame of AMI remain to be elucidated. Interestingly, autophagy induction in fly brains via Atg8a overexpression does prevent the accumulation of ubiquitinated and oxidized proteins, but has no discernable effect on AMI. Thus, in genetic terminology, promoting autophagy might not be sufficient to counteract AMI even though autophagy is obviously necessary to do so. It should be noted, though, that Atg8a overexpression does not exactly mimic autophagic induction as executed by restoring juvenile polyamine levels. Still, beyond autophagy, spermidine may trigger autophagy-independent pathways that are additionally relevant to protect against AMI. Among them might be the maintenance of memory-promoting factors whose levels decline during age. In fact, many “memory genes” (e.g., the cAMP specific phosphodiesterase Dunce) are transcriptionally induced in brains of spermidine-fed flies. The availability of these gene products might become rate limiting during aging, and thus their reexpression may be protective. Further autophagy-independent mechanisms may involve the direct adjustment of neuronal stimulation: endogenous polyamines— among them spermidine—acutely bind and modulate the activity of several classes of cation channels, among them potassium channels and glutamate receptors. Whether the changes in spermidine concentration evoked by spermidine feeding are sufficient to directly and acutely regulate neuronal excitability, synaptic Ca2+- influx and neurotransmission, and by this route ultimately learning and memory processes should be the subject of future analysis.
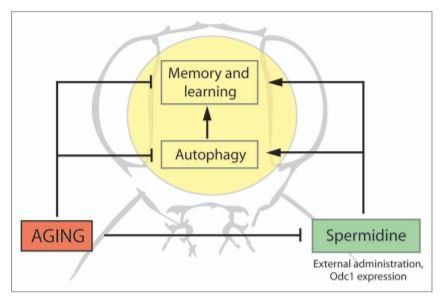
Spermidine is an endogenous metabolite that as such can be anticipated to have limited adverse side effects as opposed to non-naturally occurring or exogenous substances. This greatly augments its therapeutic potential to counteract ageassociated dementia in humans. In fact, it is conceivable that a change in dietary behavior may be sufficient to reach protective spermidine levels in the body, since polyamine-rich food elevates spermidine net levels in the blood serum. Interestingly, Natto, a Japanese food made from fermented soybeans and rich in polyamines, is considered healthy by traditional medicine, and eating Natto on a daily basis elevates polyamine levels in humans. Importantly, our data suggest that such a regimen may also be effective even when starting at an advanced age. Feeding adult flies with spermidine for only 10 d (as opposed to continuous feeding from early on) before testing at 30 d of age is sufficient to recover memory performance to nearly youth levels. Altogether, our data appear promising though, clearly, more studies are needed to test the applicability of spermidine as a strategy to contravene AMI beyond flies. Indeed, time flies and spermidine may keep us from forgetting it (Fig. 1).